We work hard to develop flexible innovative eClinical and Data Management solutions, avoiding unnecessary complexity.
Unlock the full potential of your clinical research with our intuitive and powerful EDC and CTMS solutions.
eClinical Solution for Modern Companies
Join the New Clinical Trial Experience with our software
Leading and emerging players in clinical trials
place their trust in us.
Discover how companies utilizing Datacapt are accelerating the launch of tomorrow’s innovative treatments and products.
Become a part of this change. Join them now!

Get to know our eClinical Solutions
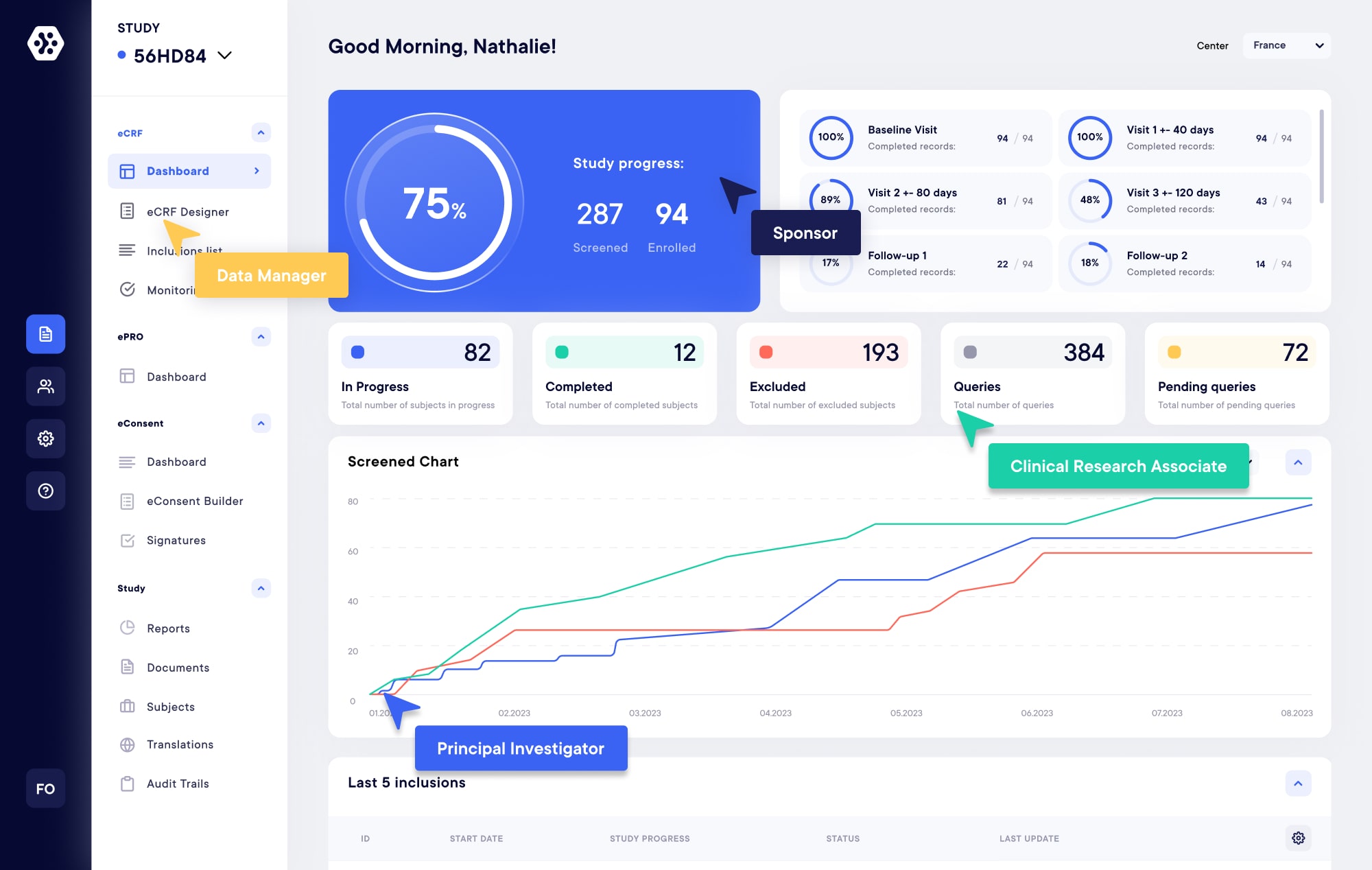
Modular and flexible solutions for your next on-site, hybrid or decentralized clinical trials. Build forms, collect, monitor, share, and manage clinical data with ease. Improve data quality and save time with our eCRF, ePRO, eConsent, Televisits, and CTMS solutions.
eCRF/eSource – EDC
Create your own studies and forms within minutes using our next-generation builder. Experience real-time data collection and monitoring from any device, at your convenience, with our modern, compliant, and flexible EDC/eCRF Platform.
eConsent
Provide an unparalleled experience while cutting down the Informed Consent process by over 50%. Initiate studies swiftly, accelerate subject recruitment, and enjoy the advantages of interactive and secure electronic consent solutions tailored to your studies.
ePRO
Eliminate paper forms and enhance subject engagement with our ePRO solution. Create surveys and gather data from individuals, whether they are sitting across the room or across the globe, utilizing various devices such as tablets, phones, and computers.
Televisits
Conduct your clinical trials either in a hybrid or fully remote manner with our eConsult and Televisit solution. Seamlessly pre-screen, screen, enroll, and evaluate participants using our modular platform for decentralized clinical trials, all integrated with our eCRF and eConsent.
Why choose Datacapt?
Streamline the management of your trial effortlessly with the Datacapt platform. Developed by specialists and experts, our platform is highly flexible and tailored to suit your specific studies. Enjoy the convenience of a modular platform that consolidates all the necessary elements in one place, enabling you to design and manage all aspects of your trials seamlessly (including EDC, eCRF, ePRO/eCOA/eDiary, eConsent, eConsult, and Data Analysis).
- Global and secure Platform (GDPR, FDA 21 CFR PART 1, HDS, ISO 27001…)
- Modern and easy to use interface
- Review and monitor your studies with real-time reports
- Quickly build your forms (eCRF, ePRO, eConsent…)
- Modular and flexible platform for simple or complex studies
- Scalable and powerful for all your projects
Enjoy a compliant and 100% secure solution






On average, our customers are more productive...
“With the Datacapt platform, we can efficiently optimize our study management while ensuring quality and safety. The rapid construction of our eCRF aligns perfectly with our procedures. Real-time monitoring of ongoing clinical trials empowers us to save time on data management and analysis, enhancing overall efficiency and confidence in our processes.”
Louis LétinierSynapse Medicine
“We chose Datacapt for one main reason: it is a modern global platform. Datacapt's advantage is its availability and responsiveness. Whenever questions or problems arise, we are confident that we will receive a quick response from them.”
Yolande JimenezComplife Group
a better clinical trials management⚬
a better clinical trials management⚬
a better clinical trials management⚬
a better clinical trials management⚬
a better clinical trials management⚬
awaits you with Datacapt⚬
awaits you with Datacapt⚬
awaits you with Datacapt⚬
awaits you with Datacapt⚬
awaits you with Datacapt⚬
Choose Datacapt for your next clinical trials.
Discover our Datacapt Platform and see how we make it easy to build, manage and conduct your clinical trials.









