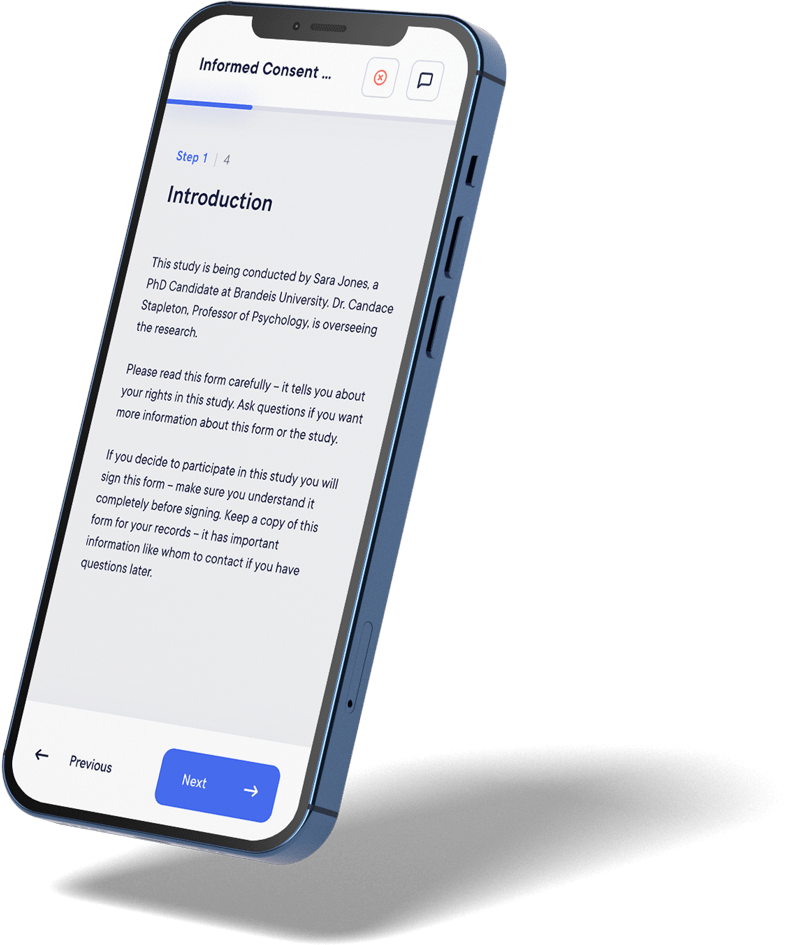
Choose an innovative digital solution that simplifies access to clinical studies, enhances patient understanding, and ensures seamless, informed, and compliant consent aligned with international standards (GDPR, FDA 21 CFR Part 11, IRB, etc.). With Datacapt’s eConsent, you boost participant engagement while streamlining your processes for greater speed and efficiency.
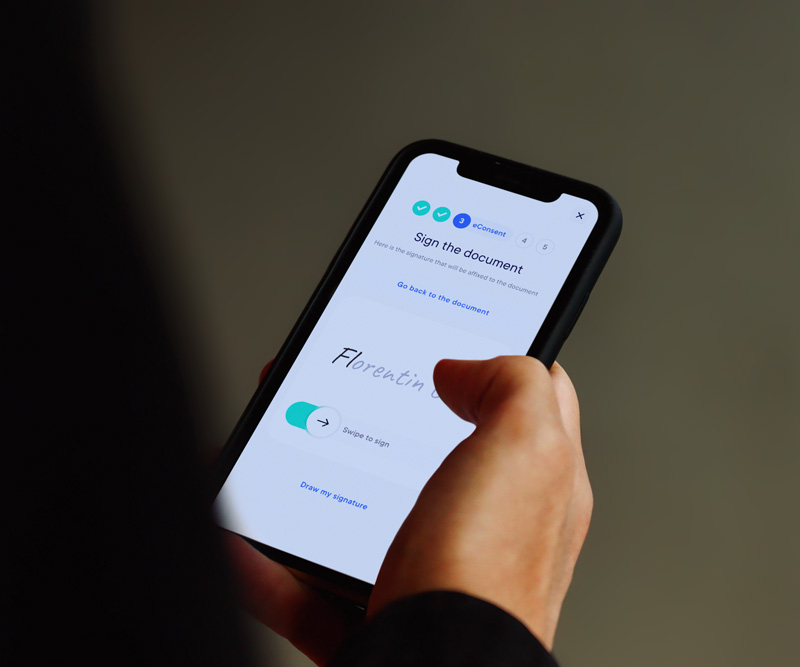
Using eConsent modernizes and enhances the informed consent process, benefiting both participants and research centers.
Best
Information
eConsent engages participants through multimedia resources, simplifying complex information. Participants can navigate at their own pace, leading to more informed decision-making.
100%
Compliant
Benefit from an eIDAS-compliant signature, ensuring identity verification, data integrity, and traceability. Collect digital consents in compliance with GDPR and ICH-GCP guidelines.
User-friendly
platform
Datacapt’s eConsent offers intuitive interfaces and guided navigation for easy use and understanding. Enjoy a straightforward, step-by-step process that is user-friendly for everyone.

Real-time visibility across all your sites
Real-time tracking and control allow investigators to guide patients through the recruitment and signing stages, ensuring compliance and proper patient information. Enhanced engagement supports regulatory adherence, fostering the success of your clinical trials.
A unique user experience
Accessible from any device (BYOD), Datacapt’s eConsent ensures a comprehensive and unique experience worldwide. Participants can easily access all your documents, ask questions, and sign effortlessly using multiple methods, enhancing accessibility and streamlining your consent process.
85+ Languages
Communicate with participants worldwide through the support of over 85 languages
Regulatory Compliance
Comply with ICH E6 R3, GDPR, IRB, and other international standards.
Multi-Channel Delivery
Share consent forms via QR codes, SMS, or email for optimal accessibility.
Multimedia Assets
Enhance understanding with videos and images integrated directly into the forms.
Legal Representative (LAR)
Enable Legal Representatives (LAR) to easily provide consent for dependent participants.
Audit-Ready
Access all detailed actions to ensure transparency and traceability.
Versions
Manage and track each version of consent documents, ensuring full participant information tracking.
Handwritten Signature
Digitize and integrate wet ink signatures into the electronic system for hybrid flexibility.

Recruitment Acceleration
eConsent provides easy access to information, signing, and tracking, reducing the workload for centers. Simplify both monitoring processes and patient recruitment.
Remote Consent
Televisits make remote electronic consent easier by allowing participants to review, receive verbal information, and sign the consent form from anywhere. Enhance accessibility and understanding while making the recruitment process more efficient.
User Management
Use flexible roles to streamline the consent process and manage multi-center studies effectively. Choose from custom or predefined roles: Administrator, Data Manager, Principal Investigator, Co-Investigator, Monitor, Read-Only Users, and more.
Compliance and Security
Specifically designed to comply with global regulations (GDPR, FDA 21 CFR Part 11, ICH GCP, etc.), eConsent digitizes and secures the consent processes to ensure accuracy, accessibility, and auditability.
Need more information?
Check out our frequently asked questions about eConsent.
Get started today with Datacapt's eConsent
Enhance understanding and recruitment for your studies with Datacapt’s eConsent.



