Join The New Clinical Trial Experience
All-in-one eClinical platform and data management solutions for modern clinical trials.
Intuitive, flexible, and 100% no-code, without the complexity of legacy systems.
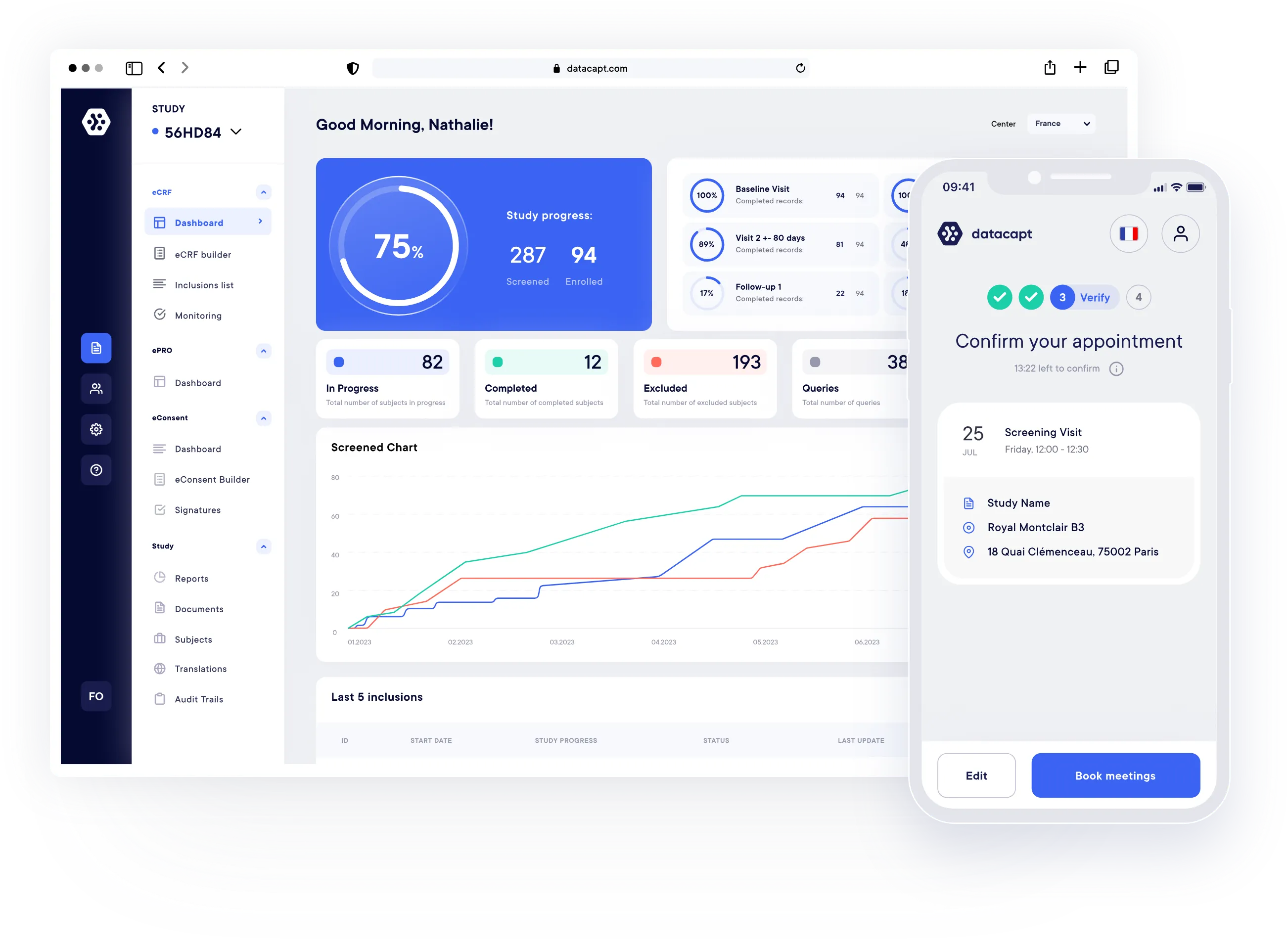
Our Solutions.


Ensure unbiased subject allocation with automated, fully integrated randomization.
Conduct secure video visits, decentralize trials, and engage patients remotely.
Automate workflows and receive real-time alerts to keep your trials on track.
Generate real-time reports and customizable metrics for data-driven decisions.
Reach global participants with support for 85+ languages.
Securely upload and centralize all trial documents for seamless collaboration.

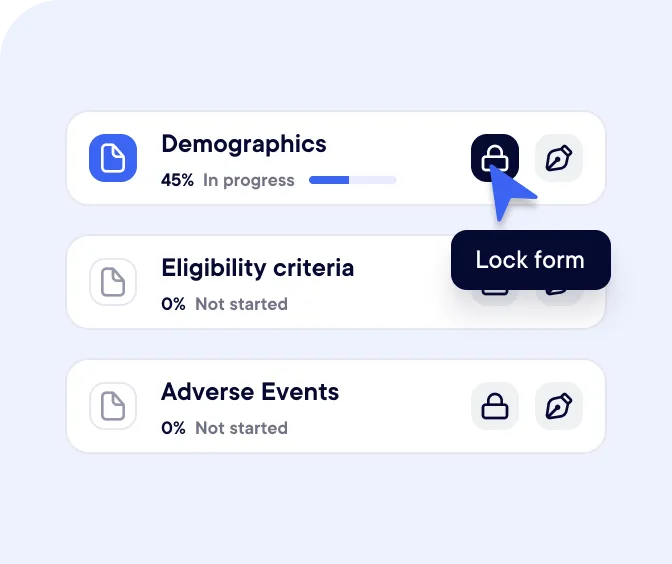
Build a flexible and powerful database with patient characteristics.
Search and filter patient characteristics based on your study criteria at a glance.
Send campaign or 1:1 messages at any time worldwide.
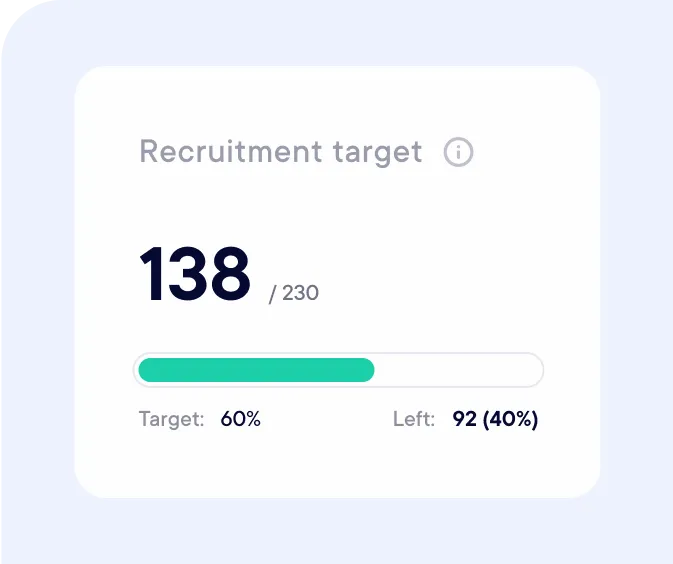
Follow your study recruitment KPIs in real-time (recruited, pending, rejected…).
Send a pre-screening questionnaire to your patients to control their eligibility.
Automatically link patients in your study to the EDC platform.
Trusted. Smart. Ready.
Datacapt revolutionizes clinical trial management, making it accessible to all industries
and enabling teams to deliver results with unmatched speed and efficiency.
Studies and +6,000 Sites
Faster (design, collect, reports)
Countries and +85 Languages
Design for Every Study,
Built for your Needs.
Datacapt
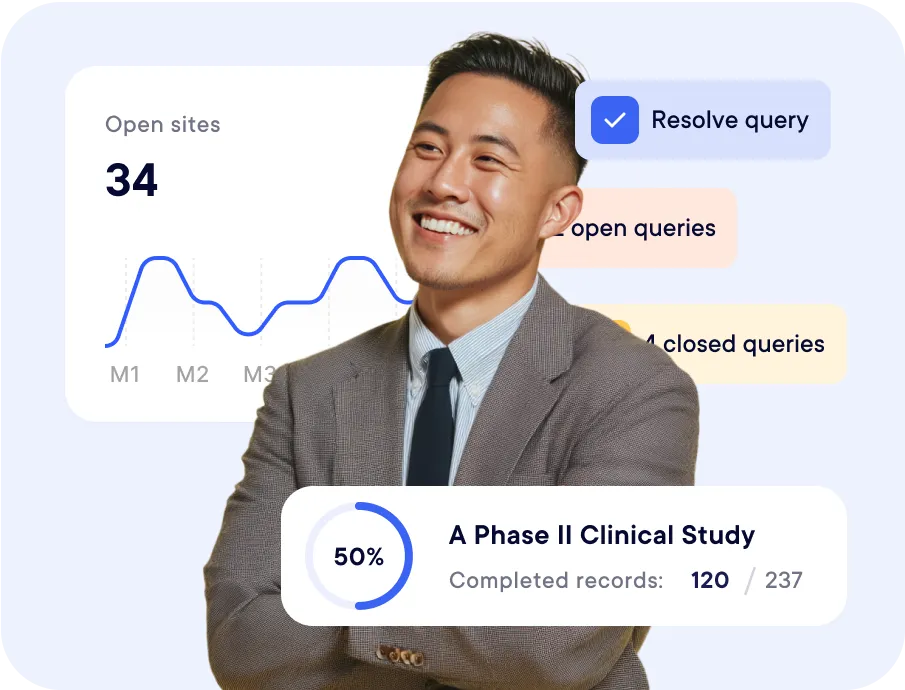
for CROs
They already use datacapt




Datacapt
for Medical Device
They already use datacapt





Datacapt
for Biotech and Pharma
They already use datacapt




Datacapt
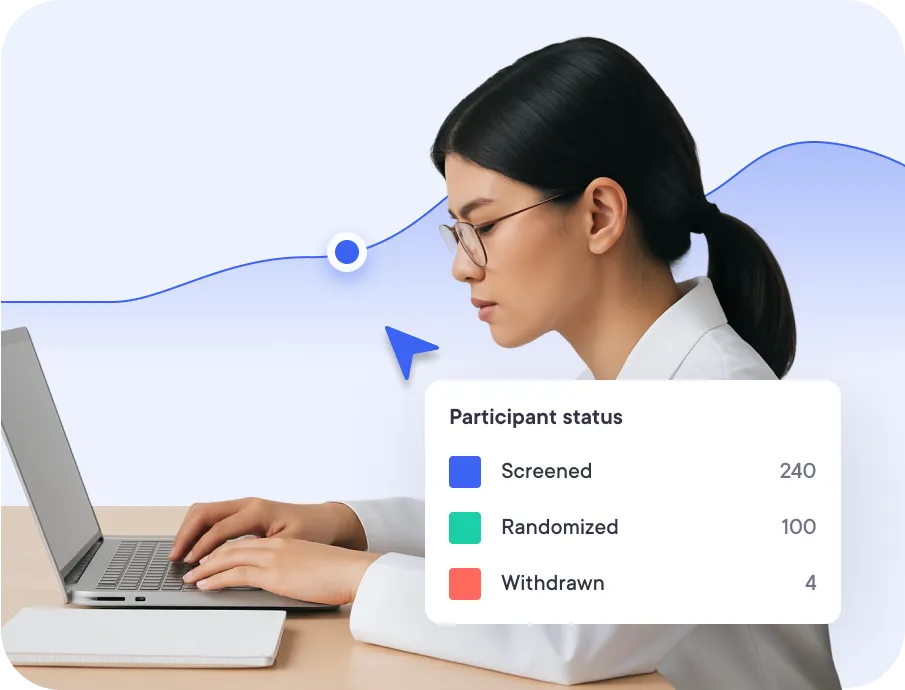
for Academic Research
They already use datacapt




Datacapt
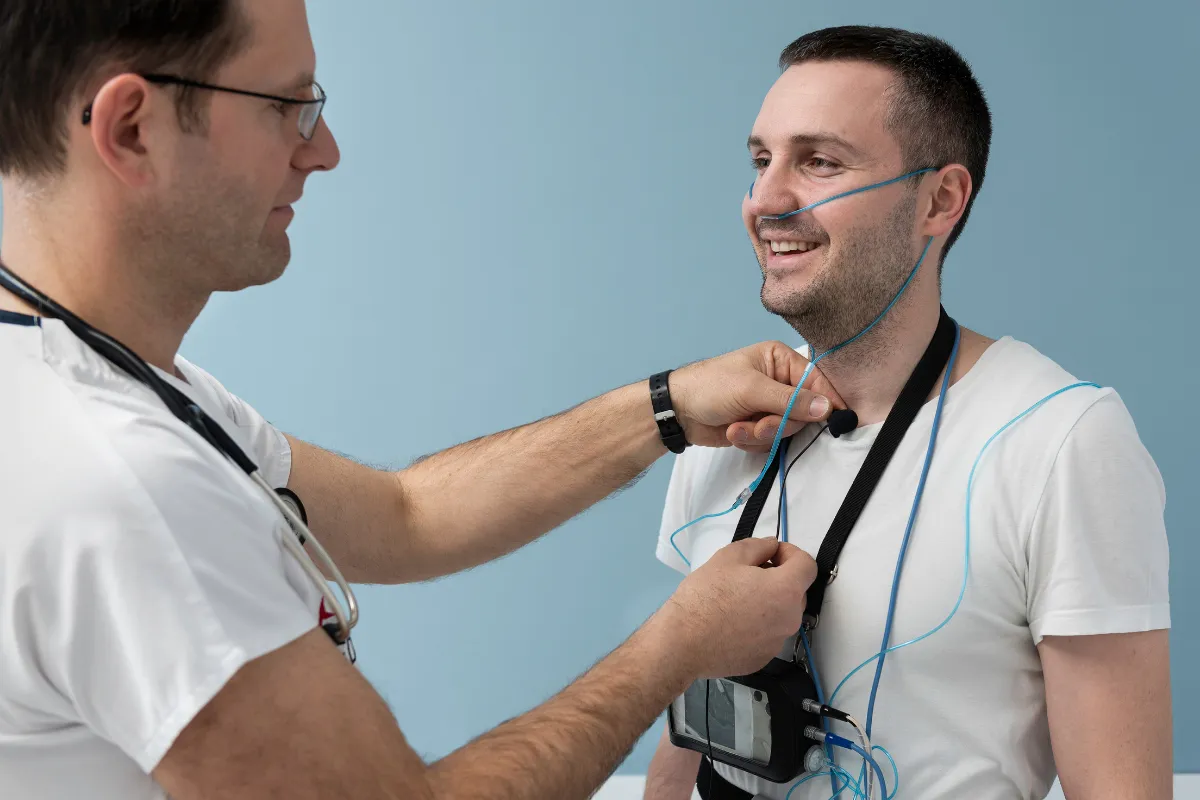
for Consumer Health
They already use datacapt
.webp)
.webp)




Datacapt surpassed our expectations thanks to its user-friendly interface and the availability of its team. It made form design and data collection remarkably intuitive resulting in significant time savings and enhanced accuracy.

Want to see Datacapt in action?
Dive into our interactive demo and explore how Datacapt simplifies every step of your clinical study from eCRF to scheduling, eConsent, reporting, and more. No jargon, no commitment, just a hands-on tour of the platform in action.
Why choose Datacapt ?
Scalable and compliant
Stay at the top of the security
.webp)

3 billion +
Data points efficiently and securely managed


99.99 %
Uptime

50 000 +
End users globally

What do customers say about Datacapt?






.webp)















.webp)









.webp)

Built for Trials.
Powered by Trust.
Experience the Difference.
Blog & News Datacapt
News, Articles, Resources et Tutorials.




















